|
I'm locked out of Twitter. Two-step verification asks me for a confirmation code from Twitter, but I never got it. Cannot find a phone number to contact a real person to speak to. Is this the future of AI-powered society? Anyone had similar experience?
0 Comments
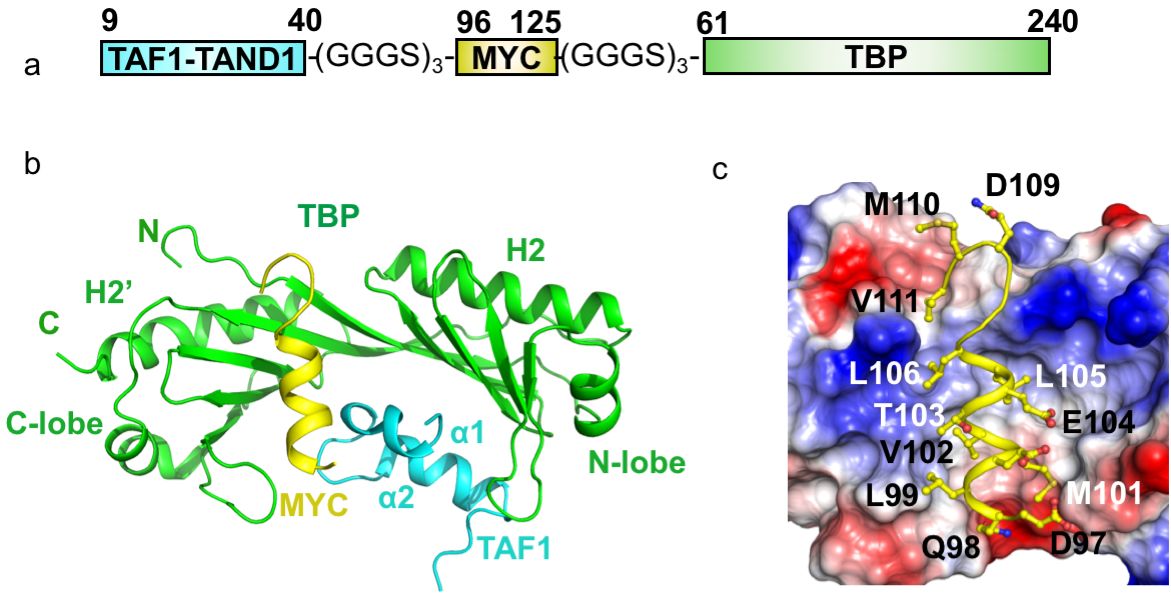
In our recent Nature Structural & Molecular Biology paper (Nov. 2019) on the interaction of the Myc oncoprotein with TATA-box binding transcription factor TBP, in collaboration with the Penn (Toronto, Canada) and Sunnerhagen (Linköping, Sweden) labs, we used a fusion protein approach to make a tripartite fusion protein that finally yielded a complex structure, which formed the cornerstone of the discovery described in the paper.
We used three copies of the GGGS motif for each linker between the target proteins. These linkers are so flexible that they did not show up in the electron density map and shall not impose any structural restraints. Several lines of evidence suggested the observed crystal structure of the fusion protein is physiologically relevant and not an artifact:
The reason we adopted this fusion protein approach is multi-faceted:
The fusion protein approach is versatile and has been used previously in other works, for example, in the structure of an Arf6 GTPase and ArfGAP(ASAP3) complex, where the affinity of the complex is low. However, this approach must be combined with other validation methods to confirm the interfaces observed in the structure are physiologically relevant. One such case of an artifact caused by the fusion approach is our unpublished structure (released in the public domain) of the fusion protein of Arf1 and ArfGAP1 (PDB:3O47), in which the interface is neither consistent with that of the Arf6/ASAP3 complex nor with an early report of the complex structure on Cell (1999). The choice of linker is also worth considering. In the PDB:3O47 structure, we used a natural intrinsically disordered peptide (IDP) region of ArfGAP1 to serve as the linker to obtain the structure. Plagiarism, including self-plagiarism, is a serious offence in academics. UWindsor Leddy library has a list of tips on avoiding plagiarism.
SafeAssign is a plagiarism checking service available for UWindsor. However, it is slightly tricky to access the service. It is available in the Blackboard Learn system. Details can be found at the Wiki-page of UWindsor Blackboard Learn. "Direct submit" in the BB Sandbox course, is a quick way to check one's writing to avoid unintended plagiarism. It is not necessary to be a course assignment to use this service. I always admired many impressive academic lab websites, such as those from my colleagues Karpowicz , Marquardt, and Porter, who are sharing the lab space with me. I finally got my website to work on an independent domain name https://tonglab.ca. Here is a short description of the journey of setting it up with the cost-effectiveness in mind. I hope it will be useful to non-tech savvy young PIs.
1. Choose an easy-to-use, professional website builder. Although I like to play with Linux a lot, I found building a website using one's server is not worth the time. Many free website builders are available to choose from. Here is a review of the top 10 choices. Wix and Weebly seem to be the two that stand out for overall performance in multiple reviews. I tried both and chose Weebly because the Weebly banner is less intrusive on the final website. 2. Build the contents. Weebly has many templates for choosing. Select one and change from there. The process is relatively straight-forward and immediately gives the website a professional look. 3. Purchase a domain name. Many domain registrars provide domain registration services. Make sure to choose an ICANN-accredited one. Despite being the most popular one with a very attractive introductory price, GoDaddy has a high renewal rate (it varies when the name gets more popular) and received some complaints about JavaScript injection. After a bit of study, I chose NameSilo, which provides a reasonable price for long-time domain maintenance. Google for "NameSilo coupon" to get a discount code. 4. Connect the Weebly site to the new domain name. In the Settings of the Weebly dashboard, change "Site Address" to the newly registered domain. Choose "connect or transfer it now" then "Connect your domain." Next, choose "Make the DNS changes your self." Here is the trick, somehow, the IP address provided by the Weebly does not work and I had to set the connection in NameSilo by choosing the Weebly DNS template for the "Website" option in the "Manage DNS" page. 5. Enable SSL in Weebly Settings. This makes sure https protocol will work, which is becoming standard for websites. 6. Log out and log in the Weebly account again. 7. Wait for about 15 minutes and for the DNS to be updated and spread throughout the Internet. Everything should be ready by now. Note: A useful tip for adding a copyright footer. Choose the "Embed Code" widget in the Build section of Weebly, place it in a web-page, and insert the following code to display the copyright footer. Replace the font name "Montserrat" to the font of your choice. <p style="font-family:Montserrat"> Copyright © 2019<script>new Date().getFullYear()>2019&&document.write("-"+new Date().getFullYear());</script> Yufeng Tong. All rights reserved. </p> I have tried quite several solutions to organize the projects I worked on, the tips accumulated, and the notes I took from seminars. The goal is to have something portable, cross-platform (different OSs, mobile), neat, efficient, easy to keep things organized, and free!
The solutions I tried include wiki-based content manage systems, MoinMoin, DokuWiki, AbstractSpoon TodoList, WorkFlowy, Microsoft OneNote, Evernote, and a few others. I ended up with Dynalist for taking personal notes and knowledge organization. I like many of the features of the free Dynalist version that meet the majority of my needs (comparison can be found here ), and it surprisingly supports some LaTeX syntax for easy math formula typesetting. Only two things that do not have a good workaround with Dynalist. First, no version control in the free version. Second, it is not suitable for collaboration, although sharing is possible. These are probably better implemented in a wiki system. |
Twitter
|
 RSS Feed
RSS Feed
